Thursday, March 27, 2008
Tuesday, March 25, 2008
Wednesday 26 March DTM week7
Slicon 1.3
We used silicon in two ways
1.We used a silicon tube sealant to attach the solar panel to the aluminium box
2.We used silicon in the actual solar pane.It converted light energy to electrical energy.
1.We used a silicon tube sealant to attach the solar panel to the aluminium box
2.We used silicon in the actual solar pane.It converted light energy to electrical energy.
aluminium 1.3
aluminium 1.3
My aluminium was cut out of a computer aided cam machine. It was cut out from a net which was made on the computer.a 2mm drilling bit was used.I then made the folds using a sheet metal bender.
Churrr
My aluminium was cut out of a computer aided cam machine. It was cut out from a net which was made on the computer.a 2mm drilling bit was used.I then made the folds using a sheet metal bender.
Churrr
Aluminium Properties 1.1 + 1.2
Pure aluminium is a silvery-white metal with many desirable characteristics. It is light, nontoxic (as the metal), nonmagnetic and nonsparking.
It is decorative. It is easily formed, machined, and cast. Alloys with small amounts of copper, magnesium, silicon, manganese, and other elements have very useful properties.
Strength depends on purity. 99.996 per cent pure aluminium has a tensile strength of about 49 megapascals (MPa), rising to 700 MPa following alloying and suitable heat treatment.
Although not found free in nature, Aluminium is an abundant element in the earth's crust.
A key property is low density. Aluminium is only one-third the weight of steel.
Aluminium and most of its alloys are highly resistant to most forms of corrosion. The metal's natural coating of aluminium oxide provides a highly effective barrier to the ravages of air, temperature, moisture and chemical attack.
Aluminium is a superb conductor of electricity. This property allied with other intrinsic qualities has ensured the replacement of copper by aluminium in many situations.
Aluminium is non-magnetic and non-combustible, properties invaluable in advanced industries such as electronics or in offshore structures.
Aluminium is non-toxic and impervious, qualities that have established its use in the food and packaging industries since the earliest times.
Other valuable properties include high reflectivity, heat barrier properties and heat conduction. The metal is malleable and easily worked by the common manufacturing and shaping processes.Physical Properties
Density / Specific Gravity (g.cm-3 at 20 °C)
2.70
Melting Point (°C)
660
Specific heat at 100 °C, cal.g-1K-1 (Jkg-1K-1)
0.2241 (938)
Latent heat of fusion, cal.g-1 (kJ.kg-1)
94.7 (397.0)
Electrical conductivity at 20°C(% of international annealed copper standard)
64.94
Thermal conductivity (cal.sec-1cm-1K-1)
0.5
Thermal emmisivity at 100°F (%)
3.0
Reflectivity for light, tungsten filament (%)
90.0
These properties can be very significantly altered with the addition of small amounts of alloying materials. Aluminium reacts with oxygen to form a microscopic (0.000000635cm) protective film of oxide, which prevents corrosion.
Aluminium in massive form is non-flammable. Finely divided particles will burn. Carbon monoxide or dioxide, aluminum oxide and water will be emitted. This is a useful property for making rocket fuel.
It is decorative. It is easily formed, machined, and cast. Alloys with small amounts of copper, magnesium, silicon, manganese, and other elements have very useful properties.
Strength depends on purity. 99.996 per cent pure aluminium has a tensile strength of about 49 megapascals (MPa), rising to 700 MPa following alloying and suitable heat treatment.
Although not found free in nature, Aluminium is an abundant element in the earth's crust.
A key property is low density. Aluminium is only one-third the weight of steel.
Aluminium and most of its alloys are highly resistant to most forms of corrosion. The metal's natural coating of aluminium oxide provides a highly effective barrier to the ravages of air, temperature, moisture and chemical attack.
Aluminium is a superb conductor of electricity. This property allied with other intrinsic qualities has ensured the replacement of copper by aluminium in many situations.
Aluminium is non-magnetic and non-combustible, properties invaluable in advanced industries such as electronics or in offshore structures.
Aluminium is non-toxic and impervious, qualities that have established its use in the food and packaging industries since the earliest times.
Other valuable properties include high reflectivity, heat barrier properties and heat conduction. The metal is malleable and easily worked by the common manufacturing and shaping processes.Physical Properties
Density / Specific Gravity (g.cm-3 at 20 °C)
2.70
Melting Point (°C)
660
Specific heat at 100 °C, cal.g-1K-1 (Jkg-1K-1)
0.2241 (938)
Latent heat of fusion, cal.g-1 (kJ.kg-1)
94.7 (397.0)
Electrical conductivity at 20°C(% of international annealed copper standard)
64.94
Thermal conductivity (cal.sec-1cm-1K-1)
0.5
Thermal emmisivity at 100°F (%)
3.0
Reflectivity for light, tungsten filament (%)
90.0
These properties can be very significantly altered with the addition of small amounts of alloying materials. Aluminium reacts with oxygen to form a microscopic (0.000000635cm) protective film of oxide, which prevents corrosion.
Aluminium in massive form is non-flammable. Finely divided particles will burn. Carbon monoxide or dioxide, aluminum oxide and water will be emitted. This is a useful property for making rocket fuel.
Silicon 1.2
Si
Composition:
Molecular Weight = 28.09 gm
Silicon 100.00 % Si
______
100.00 %
Empirical Formula:
Si
Environment:
Volcanic exhalations and minor inclusions in gold and other mantle-derived rocks. High purity synthetic material used in semiconductor manufacture.
IMA Status:
Approved IMA 1983
Locality:
Tolbachik, Kamchatka. Link to MinDat.org Location Data.
Name Origin:
From the Latin, silicis = "flint."
Synonym:
ICSD 60385
PDF 27-1402
Silicium
Composition:
Molecular Weight = 28.09 gm
Silicon 100.00 % Si
______
100.00 %
Empirical Formula:
Si
Environment:
Volcanic exhalations and minor inclusions in gold and other mantle-derived rocks. High purity synthetic material used in semiconductor manufacture.
IMA Status:
Approved IMA 1983
Locality:
Tolbachik, Kamchatka. Link to MinDat.org Location Data.
Name Origin:
From the Latin, silicis = "flint."
Synonym:
ICSD 60385
PDF 27-1402
Silicium
Silicon Properties 1.1
General
Name, symbol, number
silicon, Si, 14
Element category
metalloids
Group, period, block
14, 3, p
Appearance
crystalline, reflective
bluish-tinged faces
Standard atomic weight
28.0855(3) g·mol−1
Electron configuration
[Ne] 3s2 3p2
Electrons per shell
2, 8, 4
Physical properties
Phase
solid
Density (near r.t.)
2.3290 g·cm−3
Liquid density at m.p.
2.57 g·cm−3
Melting point
1687 K(1414 °C, 2577 °F)
Boiling point
3538 K(3265 °C, 5909 °F)
Heat of fusion
50.21 kJ·mol−1
Heat of vaporization
359 kJ·mol−1
Specific heat capacity
(25 °C) 19.789 J·mol−1·K−
Name, symbol, number
silicon, Si, 14
Element category
metalloids
Group, period, block
14, 3, p
Appearance
crystalline, reflective
bluish-tinged faces
Standard atomic weight
28.0855(3) g·mol−1
Electron configuration
[Ne] 3s2 3p2
Electrons per shell
2, 8, 4
Physical properties
Phase
solid
Density (near r.t.)
2.3290 g·cm−3
Liquid density at m.p.
2.57 g·cm−3
Melting point
1687 K(1414 °C, 2577 °F)
Boiling point
3538 K(3265 °C, 5909 °F)
Heat of fusion
50.21 kJ·mol−1
Heat of vaporization
359 kJ·mol−1
Specific heat capacity
(25 °C) 19.789 J·mol−1·K−
Monday, March 24, 2008
Tuesday, March 18, 2008
Thursday, March 13, 2008
Year 11 DTM Saturday, week 6
Week 2 And 3 Term 1 Year 11 DTM
Over these two weeks I have completed solar panel research.I worked with Jack and played around with different circuits.Overall these to weeks have been good
Week 4Thursday, 6 March 2008, 05:25 AM
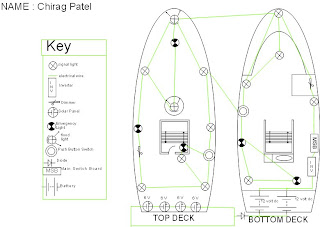
We made an engineering Drawing of our circuit. the boat design is goin well.
Week 5 Thursday, 6 March 2008, 05:41 AM
omg This week we did a free hand drawing of our boat design lol. we also did an engineering drawing of it. I have completed both sets of work (electrical) and (electronic).
tomorrow we are going on a field trip
Chirag
tomorrow we are going on a field trip
Chirag
Subscribe to:
Posts (Atom)